LATEST NEWS
ANVISA’s Latest Guidelines and Regulatory Updates for UDI Compliance
Date: 07/02/2024
In force since January 2022, RDC 591/2021, which provides for the Unique Device Identification (UDI) system, was amended by ANVISA just days before the deadline for attributing and affixing UDI to risk class IV devices. The amendment was published in RDC 884/2024 on June 1, 2024, and is already in force.
According to ANVISA, as the deadlines for adapting to the obligations stipulated by RDC 591/2021 approach, specific adjustments have been identified in the Resolution that could enhance their regulatory impact.
The new deadlines set by ANVISA for attributing and affixing UDI to medical devices, based on their risk classification and calculated from the effective date of RDC 591/2021, are as follows:
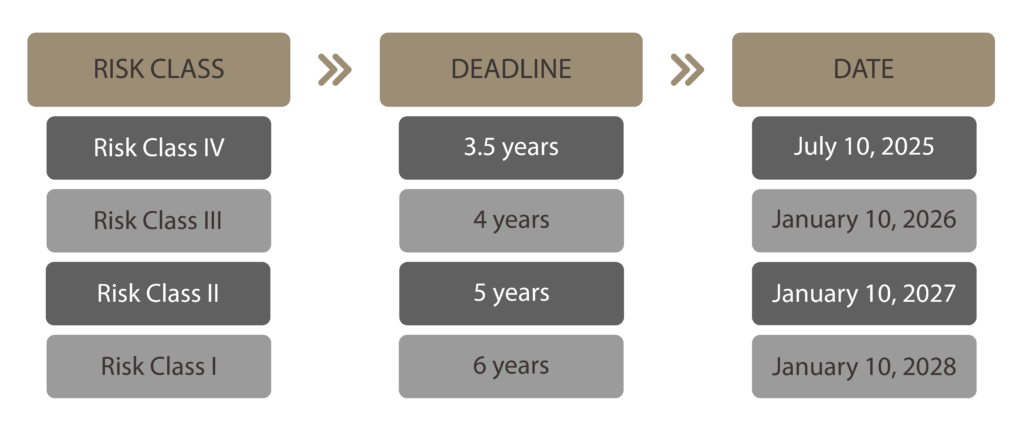
In summary, medical devices in classes II, III and IV have been granted an additional year to comply, while risk class I products retain the same previously established deadline.
ANVISA utilized RDC 884/2024 to implement specific adjustments to RDC 591/2021, including:
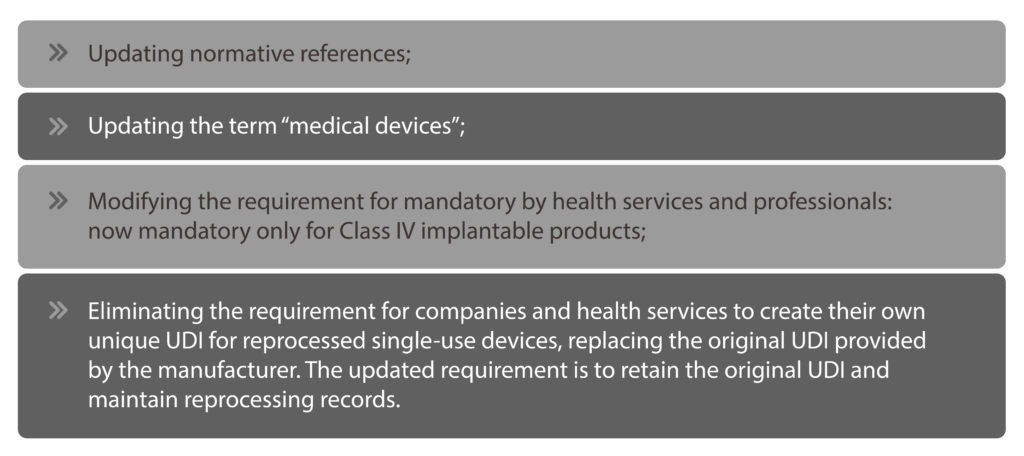
The essential data elements to be provided to the UDI database, as well as information about the UDI system, are detailed in the annexes of RDC 591/2021, which have been updated by RDC 884/2024.
The regulated sector is eagerly awaiting ANVISA’s publication of a normative instruction regarding the transmission of information to the UDI database to transmit the UDI in notifications of adverse events, technical complaints, and field actions. This normative instruction should explicitly state that ANVISA’s UDI database is fully equipped to receive and incorporate UDI information, and it should outline the procedures for data submission.
These adjustments offer manufacturers and stakeholders more time to ensure compliance with UDI requirements, thereby facilitating smoother integration and alignment with regulatory standards. This extension acknowledges the complexities involved in adopting UDI systems and aims to enhance the effectiveness and readiness of the healthcare sector in Brazil.
For updates on key developments in the Brazilian regulatory system, follow us on social media.
DOMO Salute Team
by Ângela Soska
Updated on July 4th to reflect ANVISA’s correction of the Resolution number from RDC 886/2024 to RDC 884/2024.


