LATEST NEWS
Using the MDSAP Program to enter the Brazilian Market
Date: 02/20/2020
The Medical Device Single Audit Program (MDSAP) is a program developed by IMDRF (International Medical Device Regulators Forum) that allows the conduct of a single regulatory audit of a medical device manufacturer’s quality management system that satisfies the requirements of multiple regulatory jurisdictions.
Audits are conducted by Auditing Organizations authorized by the participating Regulatory Authorities to audit under MDSAP requirements. The audit is performed based on ISO 13485, plus the country in which the certification is being applied specific regulatory requirements. The MDSAP is a way through which medical device manufacturers can be audited once to be in compliance with the standard and regulatory requirements of up to five different medical device markets: Australia, Brazil, Canada, Japan and the United States. The main goal is to promote more efficient and flexible use of regulatory resources through worksharing and mutual acceptance among regulators while respecting the sovereignty of each authority.
Since 2017 the Brazilian medical device market regulator ANVISA recognizes Auditing Organizations and up to now a sum of 13 bodies are officially authorized to perform audits that include Brazilian GMP’s requirements in their scope. The updated list of Auditing Organizations is available here. (link in Portuguese).
A report from the end of 2019, released by ANVISA, shows a significant increase in medical device manufacturers participating in the MDSAP, reporting that 48,7% of all international GMP certificates issued by ANVISA in 2019 were through MDSAP audit. It is the largest number of participating companies since the beginning of the program, in 2015.
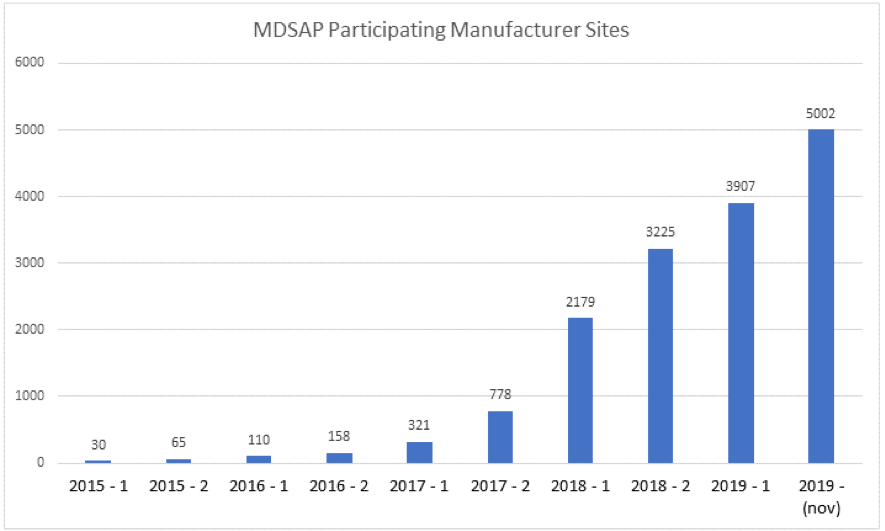
In addition to saving resources, ANVISA reports that the MDSAP program represents an increase in the health security of medical devices, as the auditing bodies inspect companies more frequently, sending reports annually to the Agency and the other authorities participating in the Program.
Doubts about joining the MDSAP Program to get a BGMP certificate? Do not hesitate in contacting our experts! Follow us on social media to get an update on the top news regarding the Brazilian regulatory system.
DOMO Salute Team


