LATEST NEWS
MDSAP Forum: Highlights Include Strategic Discussions, New Member Inductions, and Regulatory Developments
Date: 07/18/2024
From June 24 to 28, ANVISA participated in the MDSAP Forum and Regulatory Authority Council (RAC) meeting in Essen, Germany. The event gathered representatives from 27 countries, including regulatory authorities, MDSAP auditing organizations, and industry representatives, with notable attendance from Brazilian associations ABIMED and CBDL.
A highlight was welcoming a new observer member – Singapore’s Health Sciences Authority (HSA) – as well as new affiliate members: Mexico’s Cofepris, and Kenya’s Kenya Pharmaceutical Poison Board (KPPB). The RAC chair from Australia outlined MDSAP’s strategic priorities, focusing on sustainability, transparency, and efficiency, and discussed potential program expansion.
Use of MDSAP by ANVISA
ANVISA has increasingly relied on MDSAP reports to issue Good Manufacturing Practice (GMP) Certificates. The table below highlights the number of GMP certificates issued based on MDSAP reports over the years:
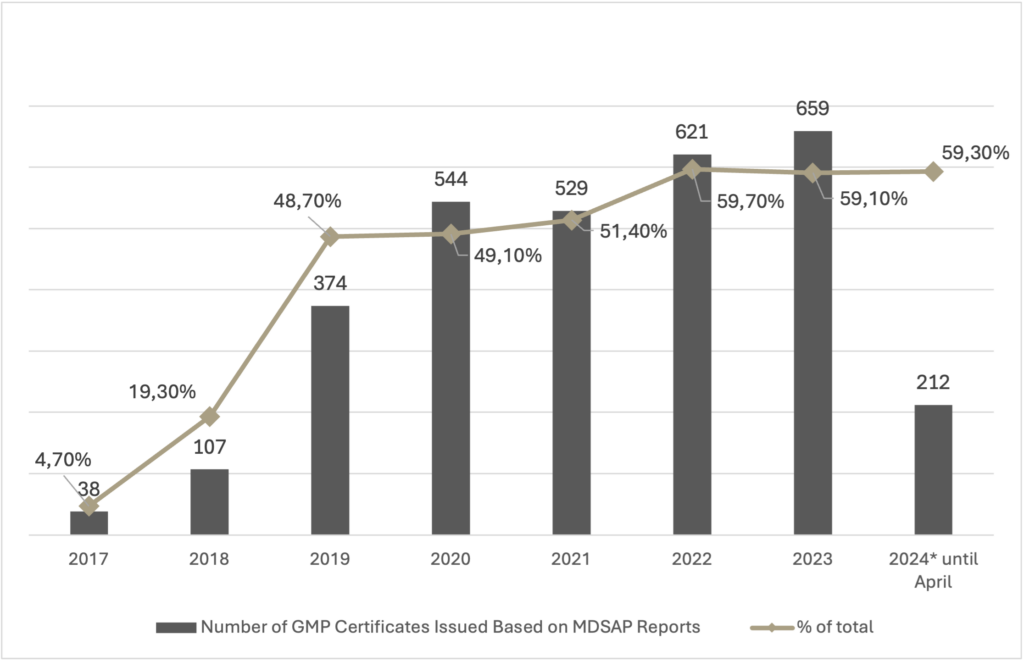
Furthermore, as emphasized in ANVISA’s presentation at the ABIMED Regulatory Journey held in May this year, ANVISA aims to achieve the goal of having 80% of issued certificates utilizing the MDSAP program. Fifteen Auditing Organizations evaluated and approved through the MDSAP program have already received ANVISA recognition. According to information provided by ANVISA at the event, approximately seven thousand companies are already participating in the MDSAP program globally.
Regulatory Development: RDC 850/2024
In March 2024, ANVISA announced a significant regulatory development with the publication of Resolution RDC 850/2024. This regulation extends the validity period of the Brazilian Good Manufacturing Practices (BGMP) Certificate from two to four years for manufacturers participating in the MDSAP.
GMP Certification Database
ANVISA offers a comprehensive GMP Certification Database designed to support effective management and oversight. This database allows users to perform searches based on relevant criteria, view geographic distributions of certifications, and filter by certification status. Updated on a weekly basis, it ensures that stakeholders have access to the most current information. The database is a valuable resource for companies and regulatory bodies to monitor compliance and maintain high standards in manufacturing practices. For more details and to access the database, visit here.
The next in-person RAC meeting is scheduled to be held alongside the International Medical Device Regulators Forum (IMDRF) in Seattle, USA, in September 2024.
For updates on key developments in the Brazilian regulatory system, follow us on social media.
DOMO Salute Team


