LATEST NEWS
Regulation for Unique Device Identification (UDI) comes into force in Brazil
Date: 01/20/2022
On January 10th 2022, the regulation that provides for the identification of medical devices regularized by ANVISA through the Unique Device Identification (UDI) system – RDC 591/2021 came into force.
The Unique Devices Identification is an international standard that complies with the International Medical Device Regulators Forum’s (IMDRF) guidance. It is defined as a series of numeric or alphanumeric characters created using a globally accepted device ID and encoding standard. These combined characters allow unambiguous identification of a particular medical device on the market.
With the beginning of the resolution’s validity, the deadlines stipulated by ANVISA for the UDI’s implementation to medical devices begins, according to their risk classification as follows:
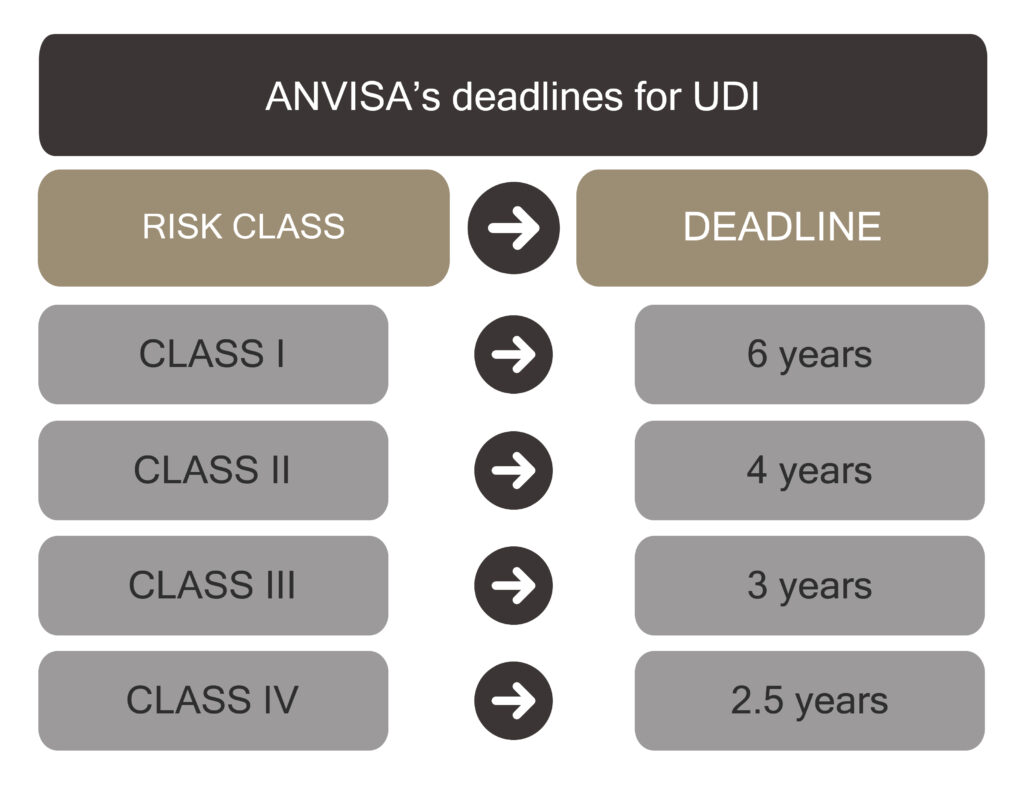
For coronary artery stents, drug-eluting coronary artery stents and implants for hip and knee arthroplasty, the inclusion of the IDU support in traceability labels is mandatory as of the effective date of this Resolution.
The essential data elements to be provided to the UDI database, as well as information about the UDI system, are included in RDC 591/2021’s annexes.
Subsequently, ANVISA will publish normative instruction regarding the transmission of information to the UDI database and the transmission of the UDI in adverse events’ notifications, technical complaints and field actions. The normative instruction shall state that the Agency’s UDI database is able to receive and include UDI information, as well as the conditions to upload data.
Follow us on social media to get an update on the top news regarding the Brazilian regulatory system.
DOMO Salute Team


